Two-Phase Heat Transfer
Nucleation superheat
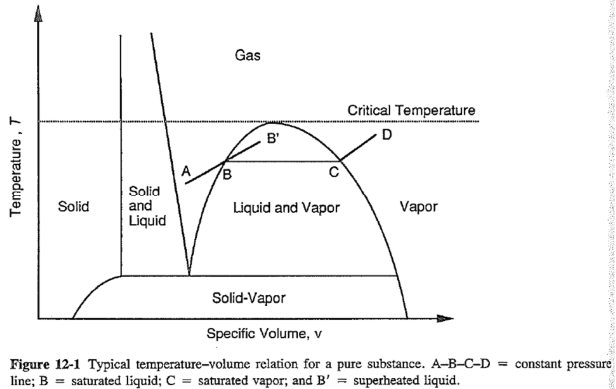
Phase change: liquid -> gas / gas -> liquid
- Homogeneous nucleation 均匀成核 (A-B')
- Heterogeneous nucleation 异相成核 (A-B-C-D)
Specific volume = density
Clausius-Clapeyron relation
Mechanical equilibrium:
: Internal pressure in bubble : Pressure outside the bubble : radius of bubble : coefficient of surface tension 表面张力
[core] Clausius-Clapeyron relation b/w
The Clausius-Clapeyron equation specifies the temperature dependencies of pressure, most importantly, vapor pressure, at a discontinuous phase transition b/w two phase of matter of a single contituent
Superheat (
when
where
Superheat for homogeneous nucleation of water at
Heterogeneous nucleation: dissolved gas & microcavities at surface
Pool boiling
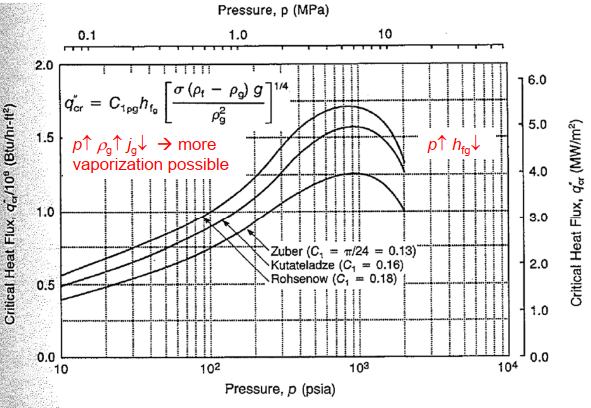
- Boiling curve: Heat-transfer regime of pool boiling
- Critical heat flux: Linked with onset of pool fluidization (suspension of liquid by vapor stream)
Hydrodynamic instability:
- Kelvin- Helmholtz instability: 2 fluid flow counterpart: critical heat flux =...
where
Flow boiling
- Heat transfer regime: Mass flow rate, fluids, geometry, heat flux magnitude and distribution
- Dry-out type CHF: High quality
- DNB (departure from nucleate boiling) type CHF (similar to CHF in pool boiling): Low quality
Enthalpy 焓 [
comes from
- The area of pipe wall:
- The whole heat:
- The enthalpy increase:
Thermal equilibrium quality at
: Saturated enthalpy - When
For boiling curve:
- single-phase flow:
- The corner:
- 2-phase flow:
Subcooled boiling
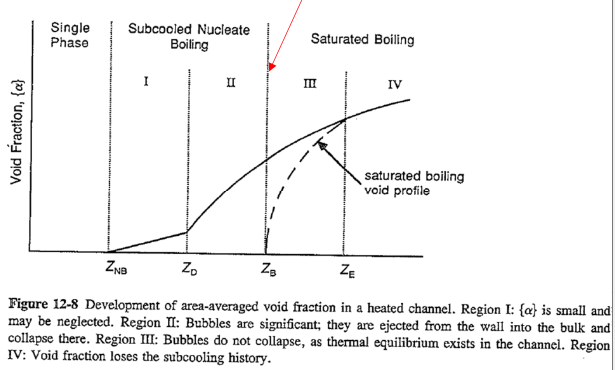
- Region I: Subcooled nucleate boiling (Onset of nucleate boiling,
: mean or bulk temperature below Tsat) - Region II: Subcooled nucleate boiling (Onset of significant void,
) - Region III: Saturated boiling (
: since liquid does not reach saturation condition due to the bubble presence and non-equilibrium) - Region IV: Saturated boiling (thermal equilibrium condition at
)
- For
, that's Saha-Zuber correlation to determine - For the curve: profile-fit method
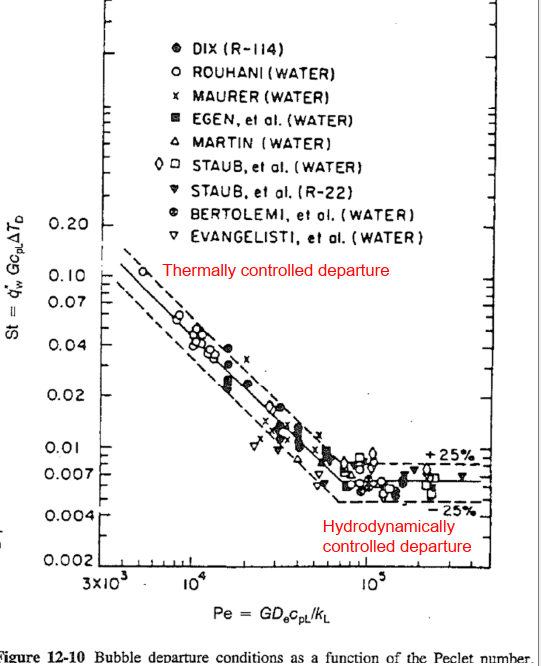
Net vapor generation (Bubble departure)
Net vapor generation: The point at which bubbles can depart from the wall before they suffer condensation (
在流体中,蒸汽的“产生”速度开始大于“凝结”速度,从而出现净增的蒸汽
- Thermally controlled departure: The wall heat flux is balanced by heat removal due to liquid subcooling at
. - Hydrodynamically controlled departure: The bubble detachment is primarily the result of drag (or shear) force overcoming the surface tension force
Saha-Zuber correlation: (empirical)
For temperature:
Subcooled flow quality and void fraction
Profile-fit approach: Currently accepted approach for determining the flow quality in subcooled region
The flow quality
at - approaches
asymptotically as increases - When
For quality:
- well-saturated boiling flow:
- Subcooled boiling flow:
Using profile-fit approach to obtain
pump
Mission: prediction of void fraction for heat transfer, etc.
- To predict the void fraction
, the Drift-flux model is used (need )
Drift-flux model:
where
and
is the flow quality
- For HEM,
and 20% overprediction
- Distribution parameter
and drift velocity can be obtained by formulas in the last chapter - To obtain the flow quality
, Profile-fit approach ( ) and OSV model (onset of significant void) are used - To obtain
, Thermal equilibrium quality is used - To obtain
, the Saha-Zuber correlation is used
- To obtain
Saturated Cooling
Heat transfer:
where is the thermal conductivity - Dittus-Boelton equation for
convective heat transfer (Turbulent flow): where
(Saturated boiling flow)
Boling incipience
Fourier's law:
Thus
Quiz3
Water is injected with a velocity of
- Calculate the mixture mass flux (unit in
). In thr uniformly heated pipe:
- Calculate the enthalpy at
from the pipe inlet (unit in ).
- Calculate the thermal equilibrium quality located at the
from the pipe inlet. Thermal equilibrium quality is calculated as
- Calculate the Peclet number for the inlet liquid.
- Calculate the temperature for onset of significant void,
, (unit in ). According to Saha-Zuber correlation, when :
Thus
- Calculate the length at which onset of significant void occurs,
, (unit in ).
- Calculate the equilibrium quality at
.
Based on Saha-Zuber correlation, when
- Calculate the quality at
. The quality is calculated based on the profile-fit approach:
where
Thus ::: details(Wrong answer in 7th leads to wrong answer here)
:::
- Calculate the distribution parameter,
, at . Given:
According to the given formula:
Wrong answer
- Calculate the void fraction at
. The void fraction is calculated by
where
Wrong answer
Thus